The reduced intensity CHOP (so-called miniCHOP) associated with CD20-directed antibody has become a standard of care for elderly patients above 80 years old, with newly diagnosed Diffuse Large B Cell Lymphomas (DLBCL)(Peyrade Lancet Hematol 2017 and Lancet Oncol 2011). While the results are consistent along with time and in several clinical trials, 40% of patients will die in the first 2 years of follow-up. Increased dose-intensity would be not achievable for most of this population, paving the way for innovative strategies. In the Relapse/Refractory setting of DLBCL, lenalidomide associated to anti-CD20 immunotherapy has demonstrated encouraging rates in both young (Houot et al. Leukemia 2019), and elderly populations (Zinzani et al. Clin Lymphoma Myeloma Leuk. 2011; Gini et al. ASH 2015) with manageable toxicities. Furthermore, the Tafasitamab combination with Lenalidomide has yeldied high and sustained response rates, with a favorable profile of tolerance (Salles et al. Lancet 2020). It is to note that frontline lenalidomide associated with Rituximab has recently been reported in a very frail population (FIL_ReRi trial, Gini et al Blood 2023), yelding an ORR of 51%.
VERLen is an open-label, international and multi-centric phase II trial designed to assess the efficacy of tafasitamab, lenalidomide and rituximab combination in very elderly patients who have a newly diagnosed DLBCL. VERLen is an Investigator Initiated study, funded by Morphosys/Incyte.
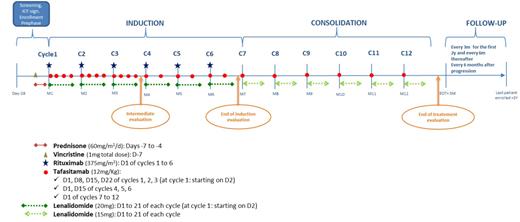
Patients must be ≥80y.o. and have a CD20+ DLBCL, with a stage I-IV PET-avid disease and a Performans status ≤2. Importantly, to achieve comparison with miniCHOP based regimens, patients should have a LVEF≥50% and no severe renal impairment (Cockroft clearance ≥30mL/min/m²). A prephase treatment with vincristine (1mg total dose) at D-7 and prednisone (60 mg/m2/d) from D-7 to D-4 is delivered prior study treatment. The treatment duration is fixed to 12 cycles (1 year). Patients receive monthly rituximab, up to 6 cycles, that can be delivered subcutaneously after 1st dose. Lendalidomide is delivered at a starting dose of 20mg/d (21d/28d per cycle) for 6 months, and tapered to 15mg/d from Cycle 7 to C12. Tafasitamab is delivered at D1,D8,D15,D22 of cycles 1-3, then D1,D15 of cycles 4-6, then monthly up to cycle 12, at a fixed dose of 12mg/kg.
Principal objective is the efficacy (overall response rate) after 3 cycles, assessed by PET-CT. We expect an increase of 15% (60->75%) of the disease control rate for the patients treated with VERLen, with a power of 90%. Seventy-one patients will be enrolled to obtain 67 evaluable patients (5% drop-out estimate). Patients with progressive/stable disease after 3 cycles should start a conventional chemotherapy (R-miniCHOP) at investigator's discretion and will remain monitored for survival. A particular attention will be given to patients that would not receive R-miniCHOP despite trial recommendation. Enrolments started in January-22, 40 patients have enrolled and Last Patient-In is awaited for Q4-2023/Q1-2024.
Secondary objectives include safety analyses, 2-y PFS/OS rates, Complete Metabolic rate at 3months ; and response/safety after R-miniCHOP for patients who switched after cycle 3. From a translational perspective, ctDNA, analysis of the microenvironnment and patients' genomics will be analysed.
CONCLUSION
VERLen trial is an innovative frontline trial for very elderly patients (≥80y.o) with newly diagnosed DLBCL, associating Tafasitamab; Lenalidomide and Rituximab, with a fixed duration of 12 cycles. Considering enrolling rates, we should provide the audience with results for ASH meeting 2024 (N+1).
Disclosures
Tessoulin:Incyte: Honoraria; Abbvie: Honoraria; Gilead: Honoraria; Kite: Honoraria. Jardin:Janssen, Gilead, AbbVie, F. Hoffmann-La Roche Ltd, BMS, Takeda: Honoraria. Morschhauser:Genmab: Consultancy, Other: Advisory Board; Epizyme: Other: Advisory Board; Novartis: Consultancy, Other: Advisory Board; Celgene: Other: Advisory Board; BMS: Consultancy, Other: Advisory Board; AbbVie: Consultancy, Other: Advisory Board; Janssen: Honoraria; Gilead: Consultancy, Other: Advisory Board; Roche: Consultancy, Honoraria, Other: Advisory Board; Incyte: Other: Advisory Board. Thieblemont:Incyte: Honoraria, Membership on an entity's Board of Directors or advisory committees; Takeda: Honoraria, Membership on an entity's Board of Directors or advisory committees; Gilead Sciences: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel Expenses; Kite: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel Expenses; Hospira: Research Funding; Bayer: Honoraria; Cellectis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel Expenses; Janssen: Honoraria, Other: Travel Expenses; Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel Expenses; Roche: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Travel Expenses, Research Funding; AbbVie: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel Expenses; Amgen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel Expenses; Paris University, Assistance Publique, hopitaux de Paris (APHP): Current Employment; Kyte, Gilead, Novartis, BMS, Abbvie, F. Hoffmann-La Roche Ltd, Amgen: Honoraria; BMS/Celgene: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Travel Expenses, Research Funding. Gros:Novartis: Consultancy, Other: Travel and accommodation expenses; BMS: Consultancy; Milteny: Consultancy; Gilead: Consultancy, Other: Travel and accommodation expenses.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal